Pyrite
Pyrite is a naturally occurring iron sulfide mineral, instantly recognizable by its metallic luster and pale brass-yellow color—earning it the nickname "fool's gold."
Pyrite is a naturally occurring iron sulfide mineral, instantly recognizable by its metallic luster and pale brass-yellow color—earning it the nickname "fool's gold."
The name pyrite comes from the Greek word pyr (fire), referencing its ability to create sparks when struck against metal or stone. Known since antiquity and referenced by ancient Greek and Roman scholars, pyrite has been formally described in early mineralogical literature and is recognized as a distinct mineral species by the International Mineralogical Association.
Pyrite belongs to the sulfide mineral group, defined by its chemical composition of iron and sulfur. It is classified under the Dana system as 2.8.1.1 and under the Strunz system as 2.EB.05, placing it among the most common and well-studied sulfide minerals in geology.
Pyrite typically presents as bright, metallic, pale brass-yellow crystals. Its most iconic forms are cubes and pyritohedrons, though it can also appear as octahedrons or massive granular aggregates. The mineral is opaque, with a strong metallic luster and a distinctive black to greenish-black streak. Specimens often feel heavy for their size due to pyrite's notable density.
Pyrite interacts with the environment by oxidizing in moist conditions, which can result in the formation of iron oxides and sulfuric acid. Historically, its spark-producing ability made it useful for igniting fires. Industrially, pyrite has served as a source of sulfur and sulfuric acid, and was once used in iron sulfate production. Today, its striking appearance makes it a favorite among mineral collectors.
Bring this kind into your world � illustrated posters, mugs, and shirts.

Archival print, museum-grade paper
Buy Poster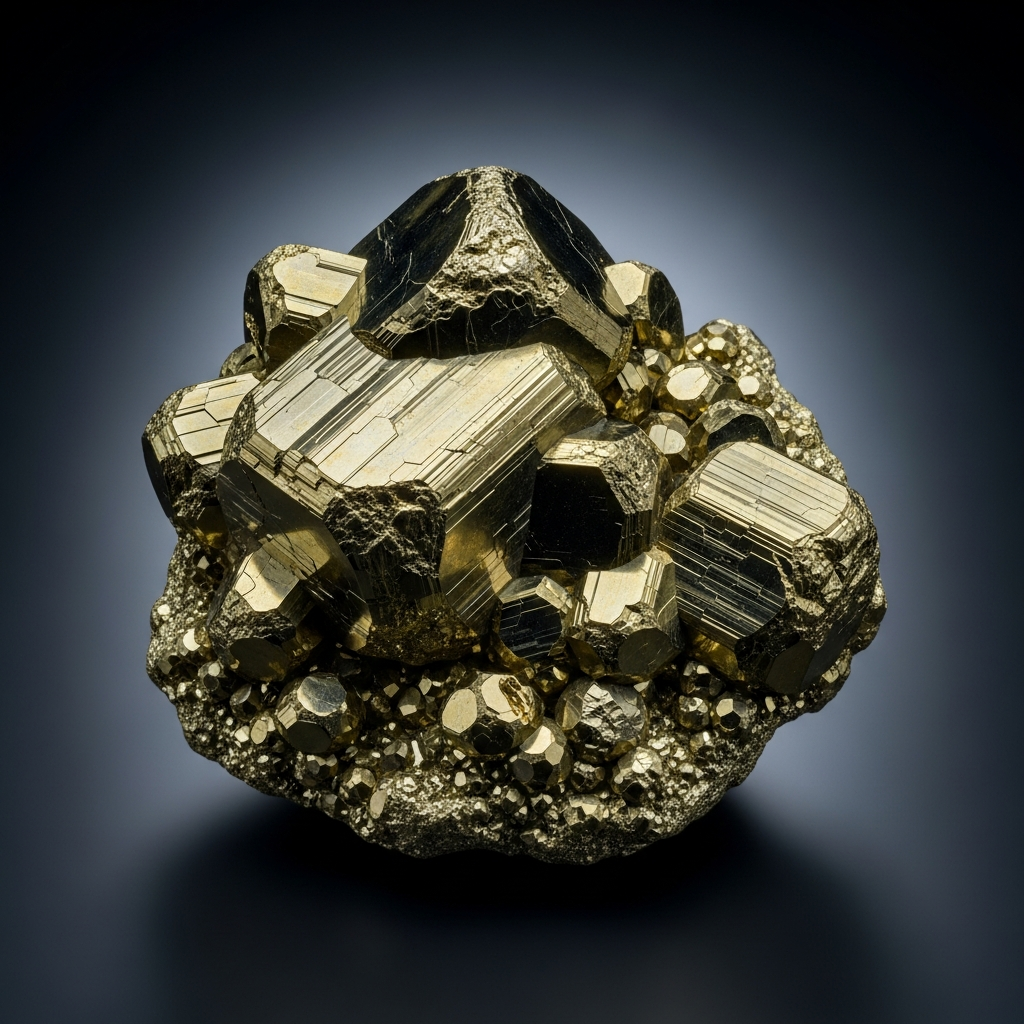
Stoneware mug, dishwasher safe
Buy Mug
Soft cotton tee, unisex sizes
Buy ShirtPyrite's resemblance to gold has woven it into folklore and popular culture as "fool's gold," symbolizing mistaken wealth and the allure of superficial appearances. It has been referenced since ancient times in literature and art, and continues to fascinate collectors and enthusiasts for both its beauty and its historical role in human ingenuity.
Pyrite is composed of iron and sulfur, with the chemical formula FeS₂ (iron disulfide).
Pyrite crystallizes in the isometric (cubic) crystal system, forming cubes, pyritohedrons, and occasionally octahedrons. Its highly symmetrical structure contributes to its sharp, geometric crystal shapes.
Pyrite is widespread in igneous, metamorphic, and sedimentary rocks. It is commonly found in hydrothermal veins, coal beds, and as a replacement mineral in fossils. Major localities include Navajún (Spain), Peru, Colorado and Illinois (USA), and Canada. Pyrite is often associated with minerals such as quartz, galena, sphalerite, and chalcopyrite.
Pyrite has been used industrially as a source of sulfur and sulfuric acid, and historically in the production of iron sulfate. Its ability to generate sparks made it valuable in early fire-starting tools. Today, pyrite's commercial use is limited, but it remains popular among collectors and is occasionally used in jewelry and decorative objects.
Store pyrite specimens in a dry environment to prevent oxidation, which can lead to the formation of iron oxides and sulfuric acid. Avoid prolonged exposure to moisture. Clean gently with a soft brush; avoid harsh chemicals or acids. Handle with care, as pyrite is brittle and can fracture easily.
Some of the world's most celebrated pyrite specimens come from Navajún, Spain, where large, perfectly formed cubic crystals are found. These specimens are highly prized by collectors for their geometric perfection and luster. Pyrite also occurs as striking replacements in fossils, creating "pyritized" ammonites and other ancient life forms.