Fluorite
Fluorite is a vividly colored mineral prized for its cubic crystals, remarkable fluorescence under ultraviolet light, and essential role as the chief source of fluorine.
Fluorite is a vividly colored mineral prized for its cubic crystals, remarkable fluorescence under ultraviolet light, and essential role as the chief source of fluorine.
Fluorite’s journey in mineralogy began in 1530 when Georgius Agricola described it as "fluorspar." Its name, rooted in the Latin "fluere" (to flow), reflects its historic use as a flux in metal smelting. Over centuries, its chemical and crystallographic identity was refined, culminating in formal recognition by the International Mineralogical Association. Today, fluorite’s legacy is woven into both industrial history and mineral collecting lore.
Fluorite is classified as a mineral species within the broader taxonomy of naturally occurring inorganic solids. It is recognized by the IMA and placed in the halide class, with Dana code 09.04.01.01 and Strunz code 3.AB.25. Its defining features are its chemical composition (CaF₂) and its isometric (cubic) crystal system, distinguishing it from other mineral groups and varieties.
Fluorite typically forms striking cubic or octahedral crystals, often transparent to translucent. Its color palette is exceptionally diverse, ranging from colorless to deep purples, blues, greens, yellows, and even banded varieties. The mineral’s surface gleams with a vitreous luster, and perfect octahedral cleavage produces smooth, mirror-like planes. Specimens may display vivid fluorescence, glowing blue-violet or other hues under UV light.
Fluorite’s physical and chemical properties make it invaluable in both industry and science. It acts as a flux in steelmaking, facilitates the production of hydrofluoric acid, and is used in optical components thanks to its transparency and low refractive index. Its fluorescence is a subject of scientific study and a delight for collectors, while gem-quality crystals are fashioned into ornamental stones.
Bring this kind into your world � illustrated posters, mugs, and shirts.

Archival print, museum-grade paper
Buy Poster
Stoneware mug, dishwasher safe
Buy Mug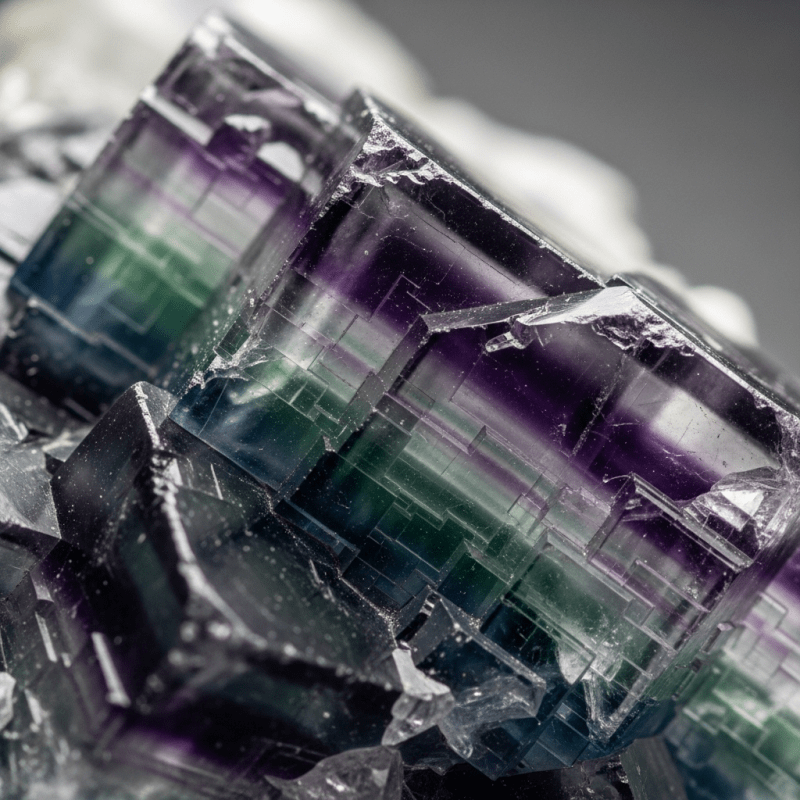
Soft cotton tee, unisex sizes
Buy ShirtFluorite has captivated collectors and scientists alike for centuries, largely due to its mesmerizing fluorescence and vivid coloration. The famed "Blue John" variety from Derbyshire, England, has been carved into decorative objects since the 18th century. Its historical role in metallurgy and its prominence in museum collections underscore its enduring cultural and scientific importance.
Fluorite’s chemical formula is CaF₂, meaning it is composed of calcium and fluorine atoms in a simple binary structure. This composition makes it the principal natural source of fluorine.
Fluorite crystallizes in the isometric (cubic) crystal system, typically forming well-defined cubes and, less commonly, octahedra. Its atomic arrangement features calcium ions surrounded symmetrically by fluorine ions, resulting in perfect cleavage along octahedral planes.
Fluorite is typically found in hydrothermal veins, often alongside minerals such as quartz, calcite, galena, and sphalerite. It also occurs in sedimentary and metamorphic rocks. Significant deposits are found in China, Mexico, South Africa, Derbyshire (England), and several U.S. states including Illinois, Kentucky, and Colorado.